Neuromuscular Electrical Stimulation (NMES) for Rehabilitation
A Neuromuscular Electrical Stimulation (NMES) device can be useful for treating muscle disuse atrophy, muscle spasms, and muscle re-education. Immobilized patients find a NMES stimulator to be useful for maintaining and strengthening muscle.
In this blog article we will review when neuromuscular electrical stimulation (NMES) may be used as an adjunct therapy modality during rehabilitation.
What Is A Neuromuscular Electrical Stimulation Device?
The FDA defines a device used for neuromuscular electrical stimulation with the following definition from 21 CFR 890.5850:
"A powered muscle stimulator is an electrically powered device intended for medical purposes that repeatedly contracts muscles by passing electrical currents through electrodes contacting the affected body area."
A muscle stimulator (identified as a powered muscle stimulator by the FDA) includes a hand-held device that acts as an interface between the pre-set therapy programs and the user. The device is connected by lead wires to electrodes that are applied to the patient's treatment site. The device transmits an electrical signal through the wires to the electrodes which passes through the skin to the nerves of the targeted muscle(s). The treatment is painless when applied correctly and poses no health risks when applied over healthy skin.
Electrical Muscle Stimulator Parameters
Many NMES devices include pre-set programs the user can choose from or will be recommended by the treating medical professional. There are 4 main parameters that define a pre-set program:
- Frequency - The frequency is measured in Hertz (Hz). Most clinical guidance places the range of useful frequency between 20 - 50Hz.
- Pulse width - This is determined by the amount of time the frequency is above a zero baseline, or below the zero baseline for biphasic or alternating current, and will define the waveform pattern.
- Ramp duration - The time it takes to reach the pre-set frequency and chosen amplitude before starting to count the pulse width is the ramp duration. This can be instantaneous or gradual.
- On/Off time - The on/off time represents when the stimulation is felt and the rest time between contractions. Muscle contraction occurs during the on time and the muscle relaxes during the off time when no stimulation occurs. The on/off time program pre-set allows the muscle to rest between contractions rather than being in a continuous contraction state. Examples of on/off time in a pre-set program are:
- 3 seconds ON, 3 seconds OFF
- 3 seconds ON, 6 seconds OFF
- 5 seconds ON, 10 seconds OFF
The amplitude (also known as the intensity) is variable and chosen by the user during a therapy session. The amplitude is increased, or decreased, based on the amount of the desired muscle contraction. The amplitude is measured in Amps or Volts based on the manufacturer's electronic circuit design and assigned a numeric scale representing the lowest setting (zero) to the maximum safe output (greatest scale number).
Some NMES devices do not include pre-set programs. These devices are set by manually selecting the desired parameters. Be certain you understand what parameters are right for your rehabilitation if you choose this type of NMES device.
What is NMES Therapy
NMES therapy is used to elicit a muscle response (contraction) by sending an electrical signal to the motor neurons. The electrical signal results in the motor neurons contracting the muscle fibers in a manner similar to voluntary muscle contractions. This is accomplished with the use of a NMES device connected by wires to electrodes applied over the target site. The stimulation of muscles is a useful adjunct treatment during rehabilitation to avoid muscle disuse atrophy, edema, muscle re-education, and spasms.
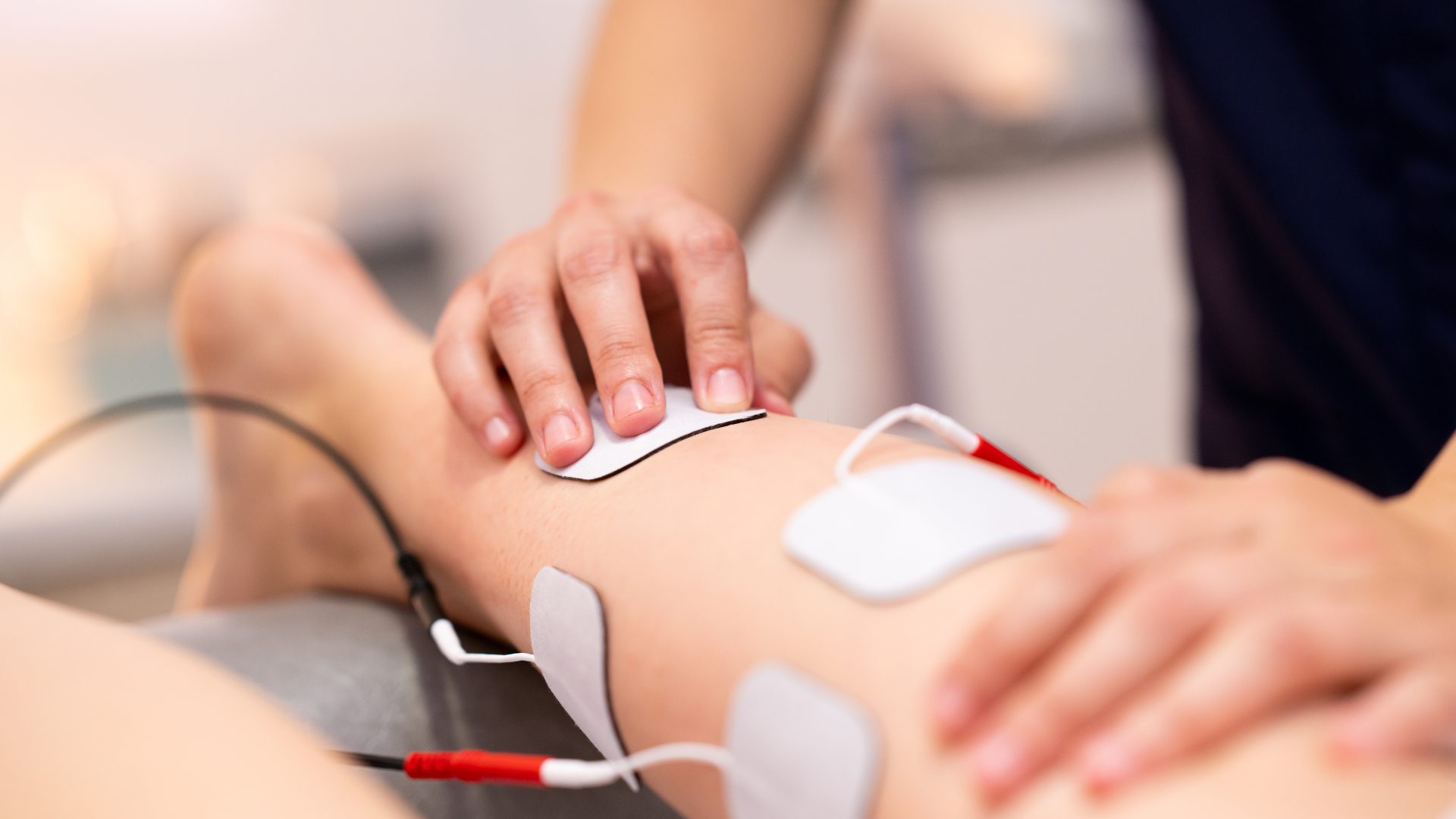
Physical Therapist applying electrodes prior to NMES treatment.
Your medical professional will determine if adding NMES therapy is appropriate for your rehabilitation process. NMES stimulation is often started before manual physical therapy such as after a cast is removed or when muscle spasms are present that prevent manual physical therapy.
Indications for Use
The FDA defines the Indications for Use of a medical device in 21 CFR 814.20(b)(3)(i) as: "A general description of the disease or condition the device will diagnose, treat, prevent, cure, or mitigate, including a description of the patient population for which the device is intended." The general indications for use that apply to a powered muscle stimulator are specific to the conditions that can be treated by the device.
Indications for use that have been approved by the FDA for powered muscle stimulators include:
- Relaxation of muscle spasms
- Prevention or retardation of disuse atrophy
- Increasing local blood circulation
- Muscle re-education
- Immediate post-surgical stimulation of calf muscles to prevent venous thrombosis
- Maintaining or increasing range of motion
Warnings
FDA warnings* for powered muscle stimulators include:
- The long-term effects of chronic electrical stimulation are unknown.
- Stimulation should not be applied over the carotid sinus nerves, particularly in patients with a known sensitivity to the carotid sinus reflex.
- Stimulation should not be applied over the neck or mouth. Severe spasm of the laryngeal and pharyngeal muscles may occur and the contractions may be strong enough to close the airway or cause difficulty in breathing.
- Stimulation should not be applied transthoracically in that the introduction of electrical current into the heart may cause cardiac arrhythmias.
- Stimulation should not be applied transcerebrally.
- Stimulation should not be applied over swollen, infected, or inflamed areas or skin eruptions, e.g., phlebitis, thrombophlebitis, varicose veins, etc.
- Stimulation should not be applied over, or in proximity to, cancerous lesions.
*Be sure to review the warnings and precautions in the user manual of your NMES stimulator before use.
Treatment Use
The medical professional overseeing your rehabilitation process will provide instructions on the frequency of use, duration, and stimulation parameters. Some muscle stimulation treatments may be performance once daily while others can be performed more than once per day.
It is recommended not to exceed the manufacturer or medical professional's recommendation for daily treatment. While unlikely but possible, you could perform too many muscle stimulation treatments resulting in excessive muscle fatigue.
We're Here to Help
The team of experts at Elite Medical Supply is here to help identify the right NMES device for your recovery. We offer a quality NMES stimulator to compliment your physical therapy and rehabilitation program. Take a look at our selection of electrical stimulators by clicking here.
When you're ready to order a brace or need assistance making a choice we're here to help. You can reach us at 866-712-0881, send us an email, or fill out a contact form.
Written by Elite Medical Supply of NY
Braces and Products Covered by Medicare
Browse ProductsRecent Posts
- Cervical Brachial Syndrome (Thoracic Outlet Syndrome): What You Need to Know
- Spinal Decompression at Home: What It Is, How It Works, and Whether It’s Right for You
- Manage Osteoarthritis Progression With a Quality Knee Brace
- Product Highlight: Aspen Active™ P-TLSO
- Understanding Radiculopathy: Causes, Symptoms, & Treatment Options
Topics
- Back Braces (39)
- Knee Braces (31)
- Medicare Beneficiaries (31)
- Pain (25)
- Non-Opioid (23)
- Medical Providers (19)
- Lower back pain (11)
- Product Highlight (11)
- Sciatica (11)
- Muscle Spasms (10)
- Decompression (9)
- Lumbago (8)
- Degenerative disc disease (6)
- Lumbar Spinal Stenosis (6)
- Spinal Stenosis (6)
- Working with your doctor (6)
- Fracture healing (5)
- Herniated Nucleus Pulposus (5)
- Bulging or herniated disc (4)
- Neuromuscular Electrical Stimulation (NMES) (4)
- Quadratus Lumborum Syndrome (4)
- SI Joint (4)
- SI Joint Dysfunction (4)
- Spondylolysis (4)
- Wrist Pain (4)
- Bone Growth Stimulator Therapy (3)
- Bone Growth Stimulators (3)
- Carpal Tunnel Syndrome (3)
- Kyphosis (3)
- Supine Cervical Traction (3)
- Unicompartmental Osteoarthritis (OA) (3)
- Wrist Brace (3)
- wrist tendinitis (3)
- Ambulatory Cervical Traction (2)
- Braces for Golf (2)
- Cervical Traction (2)
- Electrical Stimulation (2)
- Failed Spinal Fusion Syndrome (2)
- Lumbar compression fractures (2)
- Radiculopathy (2)
- Spondylolisthesis (2)
- Total Knee Replacement (2)
- Wrist Braces (2)
- Arthritis (1)
- Braces for skiing (1)
- Cervical Brachial Syndrome (1)
- De Quervain Syndrome (1)
- Digital DME Orders (1)
- Knee Brace Accessory (1)
- Knee Suspension Wrap (1)
- Knee brace support for skiing (1)
- Medicare Scam (1)
- Muscle Atrophy (1)
- PCL (1)
- Patellofemoral Pain Syndrome (1)
- TLSO (1)
- Thoracic Outlet Syndrome (1)
- Ulnar Tendinitis (1)

